
SPECTROPHOTOMETER
INTRODUCTION
TYPES
APPLICATIONS
USING GUIDE
INTRODUCTION:
A spectrophotometer is an analytical instrument used to measure the intensity of light at different wavelengths, allowing for the determination of a substance’s concentration based on its absorption of light. Modern spectrophotometers are highly sophisticated devices that utilize advanced optics and digital technology to provide precise and accurate measurements. They typically feature a range of light sources, detectors, and software for data analysis, making them invaluable tools in various fields, including chemistry, biology, and environmental science. By measuring how much light a sample absorbs at specific wavelengths, researchers can gain insights into the composition and concentration of substances, facilitating a wide array of scientific and industrial applications.
TYPES OF SPECTROPHOTOMETERS:
Spectrophotometers are essential tools in various scientific fields, enabling the measurement of light absorption, transmission, or emission by a sample. Different types of spectrophotometers are designed for specific applications, each utilizing unique principles and methodologies. Here’s an overview of the most common types:
1. UV-Visible (UV-Vis) Spectrophotometer
Introduction: UV-Vis spectrophotometers measure the absorption of ultraviolet and visible light by a sample. They are widely used in chemical analysis to determine the concentration of substances.
Explanation: This type uses a light source that emits UV and visible wavelengths, a monochromator to isolate specific wavelengths, and a detector to measure the intensity of transmitted light. By comparing the intensity of light before and after passing through the sample, researchers can calculate absorbance and, using Beer-Lambert’s Law, determine concentration.
2. Infrared (IR) Spectrometer
Introduction: Infrared spectrometers analyze the absorption of infrared radiation, providing insights into molecular vibrations and functional groups in organic compounds.
Explanation: These spectrometers typically use a Fourier Transform (FT) method or dispersive techniques. They measure the wavelengths absorbed by a sample, which correspond to the vibrational energy levels of molecules. This data helps identify molecular structures and functional groups.
3. Fluorescence Spectrometer
Introduction: Fluorescence spectrometers measure the light emitted by a sample after it absorbs light, often used in biological and medical research.
Explanation: This type involves exciting the sample with a specific wavelength of light and then detecting the emitted fluorescence at a longer wavelength. Fluorescence intensity is related to the concentration of the fluorescent species, making it useful for quantitative and qualitative analysis.
4. Nuclear Magnetic Resonance (NMR) Spectrometer
Introduction: NMR spectrometers provide detailed information about molecular structure through the interaction of magnetic fields with atomic nuclei.
Explanation: NMR relies on the magnetic properties of certain nuclei (like hydrogen) and radiofrequency radiation. When placed in a magnetic field, nuclei resonate at specific frequencies. The resulting spectra reveal information about the molecular environment, helping to elucidate complex structures in organic compounds.
5. Mass Spectrometer
Introduction: Mass spectrometers analyze the mass-to-charge ratio of ions, enabling the identification and quantification of various compounds.
Explanation: Samples are ionized and then accelerated through electric and magnetic fields. The resulting ions are sorted by their mass-to-charge ratio, producing a mass spectrum that displays the abundance of each ion. This information is crucial in fields like proteomics, metabolomics, and environmental analysis.
6. Raman Spectrometer
Introduction: Raman spectrometers utilize inelastic scattering of monochromatic light to provide molecular information, particularly about vibrations.
Explanation: When light interacts with a sample, most photons scatter elastically, but a small fraction scatters inelastically, leading to energy shifts that reveal vibrational modes. Raman spectroscopy is valuable for characterizing molecular structures and monitoring chemical reactions.
7. X-ray Spectrometer
Introduction: X-ray spectrometers analyze the emitted X-ray radiation from materials to determine their elemental composition and chemical states.
Explanation: This type uses X-ray fluorescence (XRF) or X-ray diffraction (XRD) techniques. XRF measures the characteristic X-rays emitted by elements when excited by X-ray radiation, while XRD analyzes the diffraction patterns to identify crystalline materials.
8. Atomic Absorption Spectrometer (AAS)
Introduction: AAS measures the absorption of light by free atoms, primarily used for elemental analysis in environmental and metallurgical samples.
Explanation: In AAS, a sample is vaporized in a flame or graphite furnace, and light from a specific wavelength (corresponding to the element of interest) is passed through the vapor. The amount of light absorbed indicates the concentration of the element in the sample.
9. Optical Emission Spectrometer (OES)
Introduction: OES measures the light emitted by atoms and ions excited in a plasma state, commonly used for elemental analysis.
Explanation: In OES, a sample is subjected to high temperatures (often in an inductively coupled plasma), causing atoms to emit light at characteristic wavelengths. The intensity of the emitted light correlates with the concentration of elements, making OES effective for analyzing metals and alloys.
Conclusion:
Each type of spectrophotometer has unique advantages and is tailored for specific applications, making them invaluable in research, industry, and quality control across various fields. Understanding these different types allows scientists and engineers to choose the right tool for their analytical needs.
APPLICATIONS OF SPECTROPHOTOMETE
Spectrophotometers are versatile instruments widely used across various fields for qualitative and quantitative analysis. Their ability to measure light absorption, transmission, and emission makes them invaluable in numerous applications. Here are some of the key areas where spectrophotometers are commonly employed:
1. Chemical Analysis
Spectrophotometers are extensively used in laboratories for analyzing the concentration of chemical substances in solutions. By measuring the absorbance of specific wavelengths of light, chemists can determine the concentration of unknown samples using calibration curves. This is crucial in fields such as pharmaceuticals, where precise dosage and purity are vital.
2. Biochemistry and Molecular Biology
In biochemistry, UV-Vis spectrophotometers are frequently used to measure nucleic acids (DNA and RNA) and proteins. For example, the absorbance at 260 nm is used to quantify nucleic acids, while 280 nm is used for proteins. This helps researchers assess the purity and concentration of biological samples, facilitating experiments in genetics and protein studies.
3. Environmental Monitoring
Spectrophotometers are essential in environmental science for analyzing water quality and detecting pollutants. They can measure the concentration of contaminants such as heavy metals, nitrates, and phosphates in water samples. This data is crucial for assessing environmental health and compliance with regulatory standards.
4. Food and Beverage Industry
In the food industry, spectrophotometers are used for quality control and safety assessments. They can measure the concentration of colorants, preservatives, and other additives, ensuring products meet safety standards. Additionally, they are used to analyze the nutritional content of food, such as determining the concentration of vitamins and antioxidants.
5. Clinical and Medical Applications
In clinical laboratories, spectrophotometers play a vital role in diagnosing diseases. They are used to analyze blood samples, measuring parameters like hemoglobin concentration and bilirubin levels. This aids in the detection of conditions such as anemia and liver dysfunction.
6. Pharmaceutical Development
During drug development, spectrophotometers are used to analyze the purity and concentration of active pharmaceutical ingredients (APIs). They help in studying the stability of compounds and their interactions with excipients, ensuring the efficacy and safety of medications.
7. Material Science
Spectrophotometers are employed in material science to analyze the optical properties of materials, such as polymers and nanomaterials. By measuring light absorption and reflection, researchers can study material behavior under different conditions, aiding in the development of new materials with desirable properties.
8. Forensic Science
In forensic laboratories, spectrophotometers assist in analyzing substances found at crime scenes, such as drugs, inks, and dyes. They help forensic scientists identify and quantify evidence, contributing to criminal investigations and legal proceedings.
9. Agriculture and Soil Science
In agriculture, spectrophotometers are used to analyze soil and plant samples. They help assess soil health by measuring nutrient concentrations and detecting contaminants. Additionally, they are used to monitor plant health through chlorophyll content analysis, aiding in precision farming practices.
10. Education and Research
Spectrophotometers are fundamental tools in educational institutions for teaching fundamental concepts in chemistry, biology, and physics. They facilitate hands-on learning and experimentation, helping students understand light interactions and analytical techniques.
Conclusion
The applications of spectrophotometers span a wide range of fields, from chemical and environmental analysis to clinical diagnostics and materials science. Their ability to provide precise and reliable measurements makes them indispensable in research, quality control, and various industries, contributing to advancements in science and technology.
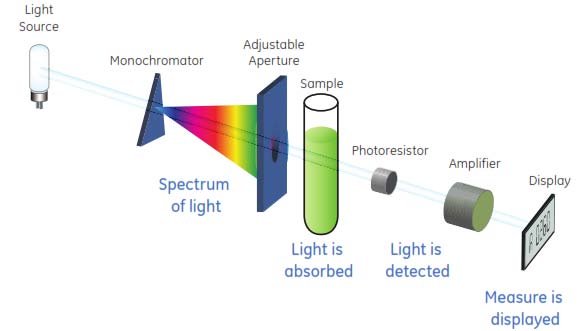
USING GUIDE FOR SPECTROPHOTOMETER:
A spectrophotometer is a versatile instrument used to measure the intensity of light at different wavelengths, often utilized in laboratories for quantitative analysis of substances. Here’s a brief guide on how to use it:
1. Preparation
- Clean the Cuvettes: Ensure cuvettes are clean and free from fingerprints or dust.
- Power On: Turn on the spectrophotometer and allow it to warm up for about 15-30 minutes for accurate readings.
2. Select Wavelength
- Choose Wavelength: Set the desired wavelength for analysis using the control panel. Refer to literature or databases for optimal wavelengths for your specific substance.
3. Blank Calibration
- Prepare a Blank: Fill a cuvette with a blank solution (solvent without analyte).
- Calibrate: Place the blank in the spectrophotometer and zero the instrument. This sets a baseline for measurements.
4. Sample Measurement
- Prepare Samples: Fill clean cuvettes with the samples to be analyzed.
- Insert Cuvette: Place the cuvette in the sample holder, ensuring it’s positioned correctly.
- Record Absorbance: Read the absorbance or transmittance value displayed on the screen.
5. Data Analysis
- Compile Results: Record the absorbance values for each sample.
- Create Calibration Curve: If applicable, plot a calibration curve using known concentrations to determine unknown concentrations.
6. Cleaning Up
- Turn Off the Device: After use, turn off the spectrophotometer.
- Clean Cuvettes: Rinse cuvettes with distilled water and dry them properly.
7. Safety Precautions
- Handle with Care: Avoid touching the optical surfaces of cuvettes.
- Wear PPE: Use gloves and goggles as necessary when handling chemicals.
Following these steps will help ensure accurate and reliable measurements using a spectrophotometer. Always refer to the manufacturer’s manual for specific operating instructions and safety guidelines.
FOR MORE DETAILS OUR SITE.